Clinical data is an important foundation for clinical research
Data management is one of the key aspects of quality control and quality assurance in clinical research. The acceptance of the results of a clinical study depends on the quality of the study data. Proper management of clinical research data ensures the integrity, reliability and accurate handling of the data, as well as the authenticity of the data.
Electronic clinical data is the trend
At present, more than 2/3 of clinical trials in the US are using EDC and the investment in EDC is increasing every year. The proportion of EDC use in clinical trials in China is also showing a yearly increase. Electronic management of clinical data seems to be the trend of clinical research development.
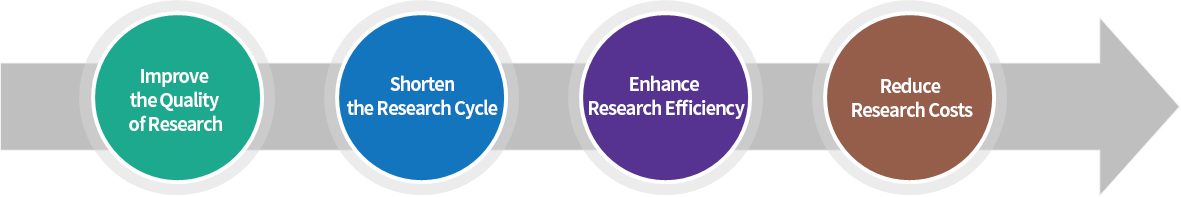
Metis accelerates the clinical trial process, reduces costs and improves quality
Metis provides an integrated EDC data management platform that changes the previous situation of slow data collection, lagging data verification, long trial cycles and poor data quality in paper-based clinical studies, resulting in faster trial processes, lower costs and higher quality.
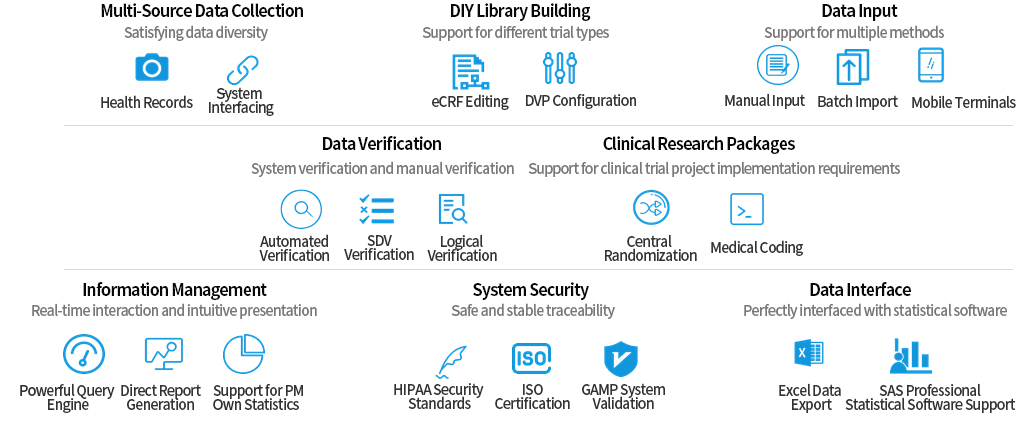

The product provides clinical researchers with an scientific approach to clinical trial design, project management concepts and efficient and convenient data management tools, based on the overall business process of clinical research.